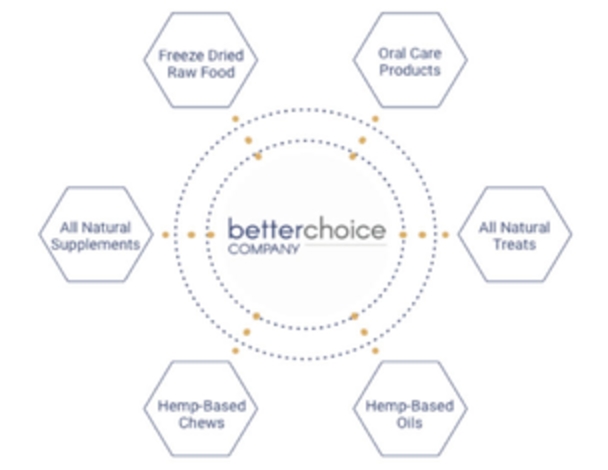
The unique attributes of our product portfolio appeal to a diverse class of consumer needs. Consequently, our brands resonate throughout a broad cross-section of pet parent demographics.
All of our products are sold under TruDog, TruCat, Rawgo!, Orapup or Hound Dog brand name, with ingredients, packaging and labeling customized by SKU.


Supply, Manufacturing and Logistics
Our products sold under the TruPet brand are sourced and manufactured in the United States, using healthy, natural ingredients. Many products are preserved using either freeze drying or gentle air dehydration to eliminate the need for artificial preservatives and added chemicals. Our treats and chews are oven-baked, using natural ingredients for maximum nutrition. TruDog raw dog foods meet AAFCO guidelines and are small-batch tested for common contaminants prior to leaving the manufacturer. The proprietary blends of our TruPet line of supplements for dogs are formulated with a focus on using natural ingredients that meet a dog’s unique biological needs.
All of our CBD soft chews and flavor-infused tinctures are formulated using pure, all natural, phytocannabinoid-rich (“PCR”) hemp-derived CBD. All CBD isolates and oils are authenticated by an independent third party via issuance of a Certificate of Analysis (“COA”), which cannabinoid content and profile, microbiological content, heavy metal content, pesticide content, and residual solvent content. We recognize the importance of compliance and partnered with one of the industry’s leading cGMP certified extraction facilities. This ensures the consistency and quality of our CBD product lines and brands.
Through its proprietary engineering process, our U.S.-based supplier isolates and removes any unwanted compounds while creating the maximum potency level of phytocannabinoids and terpenes. The cold, enclosed and continuous manufacturing processes prevent the degradation of natural molecules during extraction and purification. Made and derived from non-GMO, U.S.-grown hemp, its PCR hemp oil, and isolate powder are subjected to a rigorous testing system, both in-house and verified through independent, third party labs, which ensures accurate levels of phytocannabinoids and detects any trace amounts of THC. Our products contain only the highest level of naturally derived CBD sourced from hemp that contains less than 0.3% THC.
We believe that a key differentiator of our finished products is the quality of ingredients we source from the industry’s leading suppliers, each of whom we have carefully vetted and qualified.
Fulfillment of orders from our online customers is managed by a well-established third-party logistics partner. We utilize logistics service providers as a part of our global supply chain, primarily for shipping and logistics support. Our online ecosystem allows us to efficiently manage and customize the online shopping experience for customers,
49